What is FIBRO-VEIN 1%
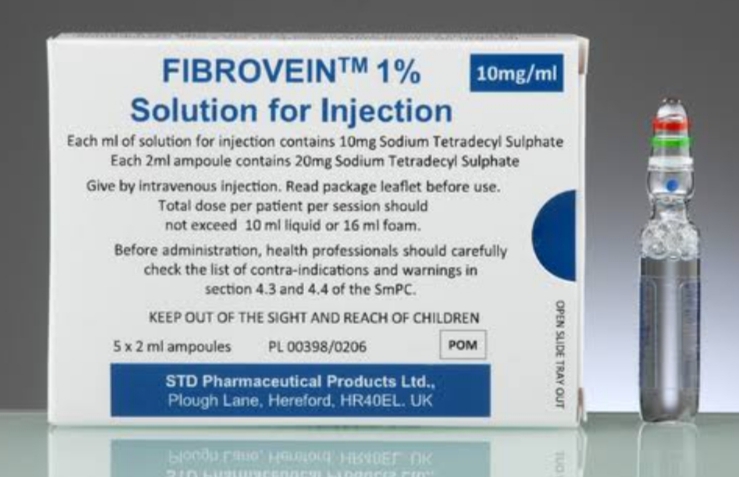
FIBRO-VEIN 1% is a sterile injectable solution that contains the active ingredient sodium tetradecyl sulfate, a synthetic surfactant used as a sclerosing agent. It is formulated for intravenous use to treat varicose veins and other chronic venous insufficiencies. Each 2 ml ampoule contains a 1% concentration, equivalent to 20 mg of sodium tetradecyl sulfate.
Therapeutic Class
It belongs to the class of medications called sclerosing agents, designed to damage the inner lining of veins, causing them to collapse and eventually be reabsorbed by the body.
Indications
- Treatment of small to medium-sized varicose veins
- Management of spider veins (telangiectasias)
- Adjunct therapy in chronic venous insufficiency
How to use FIBRO-VEIN 1%
FIBRO-VEIN 1% must be administered by a qualified healthcare provider in a clinical setting. This is to ensure correct placement and technique, as improper use can lead to serious complications.
Administration Steps
- 1. Preparation: Ensure the injection site is sterile and the patient is positioned appropriately.
- 2. Injection: Inject the medication slowly into the targeted vein using a fine needle.
- 3. Post-injection care: Apply compression to the treated area to enhance efficacy and reduce adverse reactions.
- 4. Monitoring: Observe the patient for 15–30 minutes post-procedure to manage any acute reactions.
Patient Instructions
- Wear compression stockings as advised by the physician.
- Avoid prolonged standing, hot baths, and strenuous activities for at least 48 hours post-treatment.
Mode of Action FIBRO-VEIN 1%
Sodium tetradecyl sulfate acts as a detergent that irritates the endothelium of veins upon contact. This irritation causes inflammation, leading to thrombosis of the vein and subsequent fibrosis. Over time, the occluded vein is reabsorbed and no longer visible under the skin.
Pharmacodynamics
- Destroys endothelial cells lining the vein wall
- Triggers localized clotting within the vessel
- Leads to fibrotic closure of the vein over time
Therapeutic Goal
The ultimate aim is to eliminate unsightly or symptomatic varicosities while preserving overall venous return via unaffected vessels.
FIBRO-VEIN 1% Interactions FIBRO-VEIN 1%
FIBRO-VEIN 1% generally has a low risk of drug interactions due to its local action. However, caution is warranted in patients taking systemic medications that affect vascular function or coagulation.
Notable Drug Interactions
- Anticoagulants (e.g., warfarin): May increase risk of excessive bleeding at injection sites.
- Oral contraceptives: Can raise the baseline risk for thromboembolic events.
- Other sclerosants: Avoid concurrent use to prevent overdose or cumulative vein damage.
Clinical Advice
Inform your healthcare provider about all ongoing medications, herbal supplements, and any known allergies before receiving treatment with FIBRO-VEIN 1%.
Dosage of FIBRO-VEIN 1%
Dosage depends on the size, number, and location of varicosities. It is tailored per session by the treating physician. One session can include multiple injections, and multiple sessions may be needed for complete therapy.
Typical Dosage Range
- 0.1 to 1.0 mL per injection site
- Not more than 10 mL per treatment session
- Intervals between sessions: 4–6 weeks
Clinical Notes
- Inject slowly and avoid intra-arterial administration.
- Avoid treatment of more than 10 sites in one session unless otherwise advised.
Possible side effects of FIBRO-VEIN 1%
As with any injectable medication, FIBRO-VEIN 1% can cause side effects ranging from mild and localized to systemic and rare.
Common Adverse Effects
- Pain, redness, or swelling at the injection site
- Skin hyperpigmentation
- Temporary bruising or discomfort
Rare But Serious Effects
- Allergic reaction (rash, hives, anaphylaxis)
- Tissue necrosis if injected outside the vein
- Superficial or deep thrombophlebitis
- Pulmonary embolism or deep vein thrombosis (DVT)
Monitoring
Patients should be monitored during and after treatment, especially if there is a history of clotting disorders or allergic reactions.
FIBRO-VEIN 1% Contraindications FIBRO-VEIN 1%
FIBRO-VEIN 1% should not be administered to patients with conditions that may be exacerbated by vein occlusion or who are at heightened risk of adverse vascular events.
Absolute Contraindications
- Known hypersensitivity to sodium tetradecyl sulfate
- Active thromboembolic disorders (e.g., DVT)
- Severe arterial insufficiency or ischemia
- Local or systemic infection
Relative Contraindications
- Pregnancy and breastfeeding (insufficient safety data)
- Immobility or bed confinement
- History of severe allergic reactions
Storage of FIBRO-VEIN 1%
Proper storage conditions are essential to preserve the stability and sterility of FIBRO-VEIN 1%.
Storage Instructions
- Store between 2°C and 25°C (36°F to 77°F)
- Protect from direct light and moisture
- Do not freeze
- Keep in original packaging until use
- Discard any unused solution according to medical waste guidelines
FIBRO-VEIN 1% features an exceptional active ingredient renowned for its potent effects, comprising Sodium tetradecyl sulfate. This powerful formulation provides a superior solution for addressing diverse health concerns. With 10mg/ml concentration and an easily manageable Injection/Solution for, it remains a preferred option for countless individuals seeking effective treatment.
Introduction
All you need to know about FIBRO-VEIN 1% .
Welcome to Dwaey, specifically on FIBRO-VEIN 1% page.
This medicine contains an important and useful components, as it consists of Sodium tetradecyl sulfate.
FIBRO-VEIN 1% is available in the market in concentration 10mg/ml and in the form of Injection/Solution for.
STD PHARMACEUTICAL PRODUCTS LTD. is the producer of FIBRO-VEIN 1% and it is imported from UK,
The most popular alternatives of FIBRO-VEIN 1% are listed downward .
-
Active Substance
Sodium tetradecyl sulfate
-
Size
-
Indications
- No indications available.
-
Type
-
Company
STD PHARMACEUTICAL PRODUCTS LTD.
Frequently Asked Questions
FIBRO-VEIN 1% should be stored according to the instructions provided by STD PHARMACEUTICAL PRODUCTS LTD..
In general, it is recommended to store FIBRO-VEIN 1% in a cool, dry place, away from direct sunlight
and out of the reach of children.
The duration of treatment with FIBRO-VEIN 1% may vary depending on the condition being treated
and the guidance of your healthcare provider. It is important to follow the prescribed treatment
plan and continue taking FIBRO-VEIN 1% for the recommended duration, even if your symptoms improve.
If you have any concerns or questions about the duration of treatment, consult your healthcare provider.
It is important to check with your healthcare provider or read the medication label for specific
instructions regarding alcohol consumption while taking FIBRO-VEIN 1%. Some medications, including
FIBRO-VEIN 1%, may have interactions with alcohol that can reduce effectiveness, increase side
effects, or pose other risks to your health. It is best to follow the guidance provided by your
healthcare professional.
If you miss a dose of FIBRO-VEIN 1%, take it as soon as you remember. However, if it is close
to the time for your next scheduled dose, skip the missed dose and continue with your regular dosing
schedule. Do not take a double dose to make up for a missed one unless advised by your healthcare provider.
No, do not stop taking FIBRO-VEIN 1% without consulting your healthcare provider, even if your
symptoms improve. It is important to complete the full course of treatment as prescribed. Stopping
the medication prematurely may lead to a relapse or incomplete resolution of the condition. If you
have concerns about the duration of treatment, consult your healthcare provider for guidance.
It is important to consult your healthcare provider before taking FIBRO-VEIN 1% if you are
pregnant or breastfeeding. They will be able to assess the potential risks and benefits based on your
specific situation. Please note that the safety and suitability of FIBRO-VEIN 1% during pregnancy
or breastfeeding may depend on the active substance [Active Substance], concentration 10mg/ml,
and the specific recommendations of STD PHARMACEUTICAL PRODUCTS LTD..
The effects of FIBRO-VEIN 1% on your ability to drive or operate machinery can vary depending on
the active substance [Active Substance], concentration 10mg/ml, and individual factors.
Some medications may cause drowsiness, dizziness, or other side effects that can impair your judgment
or coordination. It is important to read the medication label or consult your healthcare provider to
understand any potential effects on your ability to perform tasks that require alertness.
The instructions for taking FIBRO-VEIN 1% with or without food may vary depending on the medication
and the recommendations of STD PHARMACEUTICAL PRODUCTS LTD.. Some medications may be more effective when taken with
food to enhance absorption or reduce stomach irritation, while others may need to be taken on an empty
stomach for optimal absorption. Read the medication label or consult your healthcare provider for specific instructions.
The use of FIBRO-VEIN 1% in children or elderly individuals may depend on various factors, including
the specific medication, type Injection/Solution for, and the recommendations of STD PHARMACEUTICAL PRODUCTS LTD.. Some
medications may have specific dosing instructions or precautions for these age groups. Consult your
healthcare provider or read the medication label for information regarding the safe and appropriate use
of FIBRO-VEIN 1% in children or elderly individuals.
Dwaey
All medical information published on the Dwaey website aims to increase medical awareness and health education among users. However, it is not a substitute for professional medical advice. Always consult with a specialist doctor. We strongly advise against using any information or medicine found on the site without referring to your healthcare provider.
Related Products

0 Comments